
The Goode Lab at Brandeis University
Scientific Illustration
These illustrations were produced by Julian Eskin and Bruce Goode and are made available for download and use under the Creative Commons 4.0 Attribution License. They were made using a combination of Adobe Illustrator, Photoshop, and Blender software.
(Description)
(Description)

Fig. 1 Stages of endocytic patch initiation, maturation, and turnover. The stages of progression (numbered 1–9) of an endocytic patch from initiation to membrane invagination, followed by scission and disassembly/uncoating, make components available for new rounds of patch formation (recycling). Boxes describe the concurrent sequential behavior of the actin cytoskeleton and actin-associated proteins during this process. Each icon represents a group of proteins with related functions, activities, and/or localization.
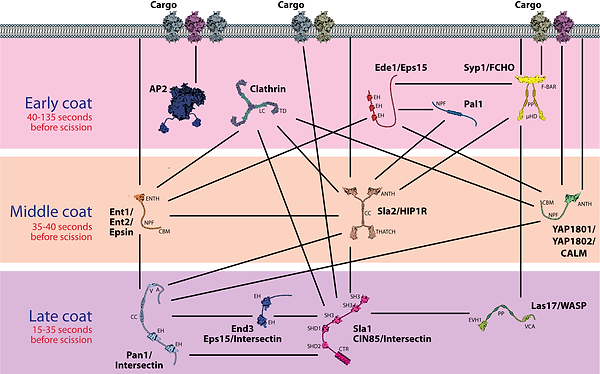
Fig 2. Endocytic adaptor and coat proteins. Depicted are the key factors in the three stages of endocytic coat formation, early, middle, and late coat proteins, which are recruited at distinct stages of endocytosis and thus represented as separate layers. Importantly, these represent temporal and not necessarily spatially segregated layers. Lines indicate known binding interactions among components.

Fig 3. A comparison of the four stages in formation and turnover of actin patches vs. cables. Scheme of the ultrastructure of a cortical actin patch (top) and cable (middle); legend defining the icons (bottom). Both structures are initiated by the nucleation of filaments from ATP-bound G-actin (left). Then, F-actin is stabilized/crosslinked and subsequently severed and disassembled. The released ADP-actin monomers undergo nucleotide exchange to replenish the pool of polymerization-competent ATP-G-actin (right side). Some icons represent groups of proteins with related activities (e.g., Arp2/3 complex NPFs, disassembly cofactors of cofilin). This cycle takes ∼5 sec to complete in each type of actin structure; due to distinct nucleation rates, it occurs within the <300 nm ribosome exclusion zone in an actin patch vs. >5000 nm along the length of an actin cable.

Fig 4. Arp2/3 complex nucleation-promoting factors (NPFs). The figure depicts three proposed stages of NPF activity. (A) Inhibited NPFs, (B) Network priming, and (C) Active nucleation.

Fig 5. Actin turnover machinery. Scheme showing the proteins that regulate actin-filament debranching, filament severing and depolymerization, and monomer recycling. Dotted lines are physical interactions. Numbers correspond to the designated steps in actin turnover in which each protein participates.

Fig 6. Structure of Cofilin bound to filament, a model for Twinfilin bound to filament side, and cartoon depictions of free Aip1, Coronin, and Srv2/CAP


Fig 7. (A) Schematic representation of four mechanisms of actin filament nucleation. Spontaneous assembly of actin monomers (G-actin) into filaments (F-actin) is kinetically unfavorable, however, actin tetramers are stable, and once formed, new subunits will readily associate with the barbed (+) end. (B) Schematic representation of teh two known mechanisms for promoting actin filament elongation. Formins processively associate with the growing barbed ends of filaments and accelerate elongation by recruiting profilin-bound actin monomers and delivering the, to the barbed end.

Fig 8. Stages of collaborative actin filament assembly by APC and formin

Fig 9. (A) Actin structures in budding yeast at different stages of cell cycle, (B) Branched actin filament networks nucleated by the Arp2/3 complex at sites of endocytosis, (C) Linear actin bundles (cables) polymerized by formins and used for intracellular traffic, (D) Actomyosin contractile ring assembled by formins at the bud neck to facilitate cytokinesis.